

In our experience, conventional Scintillation Proximity Assay (SPA) technology for HTS is limited to use in 96-well format. With the drive towards miniaturization in HTS, Amersham Pharmacia Biotech have introduced the LEADseekerTM imaging system and LEADseekerTM imaging beads for enabling miniaturized scintillation proximity assays (SPA). These new beads are either polystyrene or yttrium oxide and can be obtained with a variety of coatings. Evaluations of all bead types in a variety of biochemical assays have demonstrated that the yttrium oxide beads are applicable to more miniaturized assay types, as they produce a higher signal and have a lower background phosphorescence. Unfortunately, robust dispensing of yttrium beads is very difficult due to their high density (5g/cm3), five times that of the polystyrene beads. This results in very rapid settling if the beads are not agitated continuously. This presents some difficult liquid handling challenges if robust and reliable low volume dispensing is to be achieved.
We have successfully demonstrated accurate and precise bead dispensing of yttrium oxide beads in the 1-4 µL volume range into both low volume 384- and 1536-well plates using the Cartesian synQUADTM technology. We have used this dispensing technique to validate with a 10 µL LEADseekerTM assay in a proof-of-concept study.
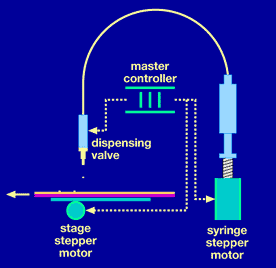
The Cartesian Technologies (Irvine, CA) SQ series dispensing workstation is a high-speed dispensing platform for delivering nanolitre to microlitre volumes of chemical and biological reagents. The workstation uses proprietary synQUADTM technology, a synchronised version of Cartesian's widely used non-contact quantitative microdispensing technology. This combines the high-speed actuating capability of a micro-solenoid inkjet valve with the high-resolution displacement of a syringe pump. By synchronising the valve, the pump, and the x-y positioning system, synQUAD technology provides high-speed quantitative dispensing of nanolitre to low microlitre volumes (20 nL to 8 µL) in a non-contact mode. Dispensing is accomplished by moving the dispense head above the microtitre plates and dispensing drops "on-the-fly". (See Fig. 1.1 and Fig. 1.2 below.)
 |
 
|
| Fig 1.1. Schematic of the synQUAD technology. | Fig 1.2. An 8 channel synQUAD SQ3200. |
AIM
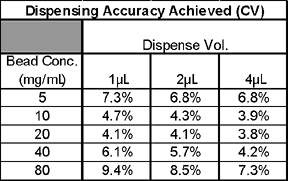
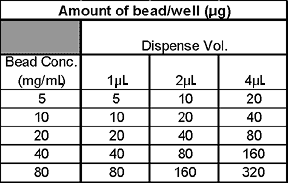
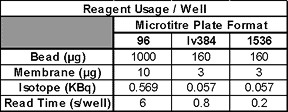
We have assessed the ability of the synQUADTM to dispense yttrium oxide (YOx) beads into 384- and 1536-well plates and to provide an accurate, rapid and robust solution for low volume bead dispensing. It was felt that in order for the instrument to be of maximum use for screening LEADseekerTM assays in the 50 to 10 µL assay volume range we would need to demonstrate YOx bead dispensing up to 300 µg per well. In order to achieve this, we dispensed 1, 2 & 4 µL volumes at bead concentrations of 5, 10, 20, 40 and 80 mg/mL.
For bead dispensing the synQUAD would be used in aspirate and dispense mode, aspirating the bead solutions from a stirred pot and then dispensing to the assay plate.
For this study we dispensed 3H-biotin linked to streptavidin-coated yttrium oxide LEADseeker beads and then imaged them using the Amersham Pharmacia Biotech LEADseekerTM -103° CCD imager.
DISPENSE CYCLE
The protocols contain all the settings that were used to evaluate the synQUAD with ceramic tips and a 5 µL syringe. The structure of the aspirate and dispense cycle was identical for all volumes:
1 µL = 4 passes of 24 1 µL drops
2 µL = 2 passes of 24 2 µL drops
4 µL = 1 pass of 24 4 µL drops
(NOTE: For all volumes 96 µL of beads are dispensed.)
RESULTS
 |
 |
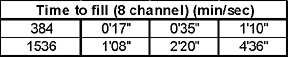 |
The results demonstrated that the dispensing accuracy was well within acceptable limits (<10% CV) for all of the bead concentrations and volume ranges. The synQUADTM also demonstrated considerable robustness with only one missed well being observed in the entire experiment (~19,000 wells).
 |
 |
|
Fig 2.1. Greiner 1536 MTP filled with 4 µL @ 40 mg/ml. |
Fig 2.2. Greiner Low-Volume 384 MTP filled with 2 µL @ 20 mg/mL. |
AIM
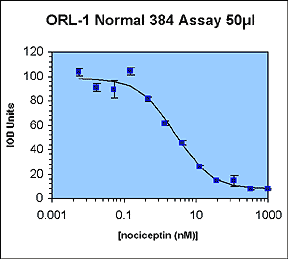
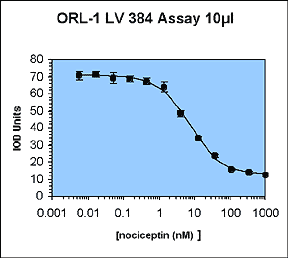
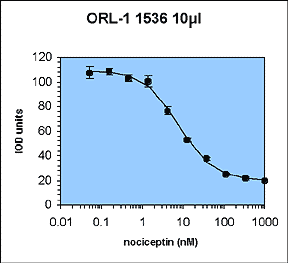
The aim of this work was to perform a 10 µL automated LEADseekerTM assay to validate all of the aspects of liquid handling and imaging associated with running low-volume assays.
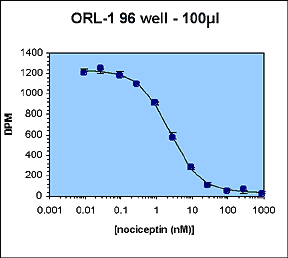
A previously existing binding assay for the orphan opioid receptor ORL-1 was chosen. This 96-well homogeneous scintillation proximity assay measures agonist binding of 3H-nociceptin to wheat-germ agglutinin-PVT SPA beads and recombinant ORL-1 membrane preparations.
The assay was suitable for two principle reasons. Firstly, it had already been run routinely as a 96-well SPA, and we had fully characterised the assay development and automation issues. Secondly, the assay is tolerant of 10% DMSO, and so we can use conventional pipetters to perform a 1 µL compound addition into 10 µL assay.
ASSAY PROCEDURE FOR 10 µL ASSAY
The assay was performed at 10 µL in Greiner low-volume 384- and 1536-well white plates. The procedure and equipment used was:
RESULTS
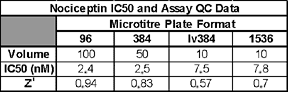
The IC50 and Z' values obtained are all within the acceptable range.
 |
|
|
|
|
|